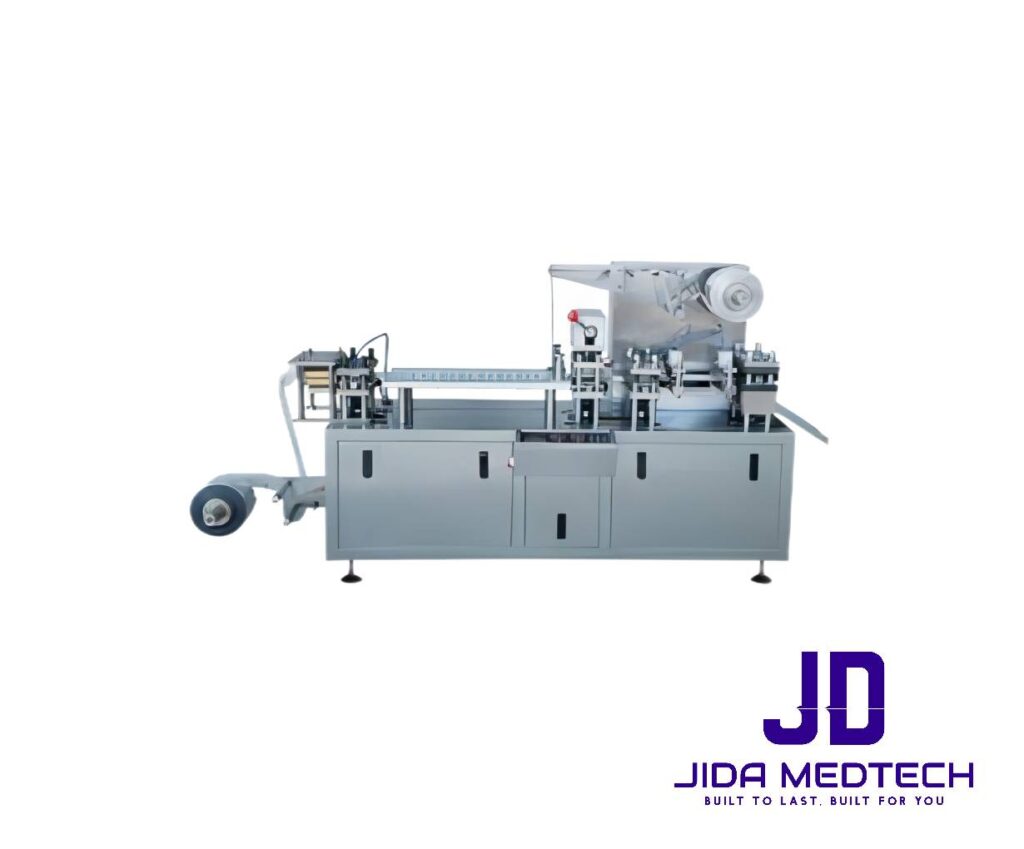
Model: JDM-BP001
Productivity: 1000-3000 pcs per hour
Travel: 30-90 mm
Max. size: 140x110x25 mm
Power: 3.5kw, 220v
Dimension:
Introduction: Packaging endodontic NiTi (Nickel-Titanium) files using a blister packaging machine involves several steps to ensure the sterile and secure containment of the instruments. Here is a general guide on how to pack endo NiTi files using a blister packaging machine:
1. Preparing the Blister Packaging Machine:
- Ensure the blister packaging machine is clean and properly calibrated.
- Set the machine to the appropriate specifications for the size and type of endodontic NiTi files being packaged.
2. Loading the Blister Material:
- Insert the blister material into the machine. This material is typically a clear, flexible plastic sheet that will form the front of the blister package.
3. Placing the Endo NiTi Files:
- Position the endodontic NiTi files onto the blister material in the designated cavities or pockets.
- Ensure that the files are properly aligned and spaced to prevent any damage during the sealing process.
4. Adding the Backing Material:
- Place the backing material, which is usually a cardboard or foil material, over the endodontic NiTi files. This backing provides support to the files and seals the package.
5. Sealing the Blister Package:
- Activate the blister packaging machine to seal the edges of the package.
- The machine typically uses heat or pressure to bond the blister and backing materials, creating a sealed compartment for the endodontic NiTi files.
6. Trimming Excess Material:
- Trim any excess material around the sealed edges to create a neat and uniform package.
- This step ensures that the package is visually appealing and easy to handle.
7. Printing Batch Information:
- If required, use the blister packaging machine to print batch information, expiration dates, or other essential details on the packaging.
- This information is crucial for traceability and compliance with regulatory requirements.
8. Quality Control Inspection:
- Conduct a quality control inspection to ensure that each blister package is properly sealed, and the contents are secure.
- Check for any defects or abnormalities in the packaging.
9. Sterilization (if necessary):
- If the endodontic NiTi files require sterilization, this step typically occurs before or after the blister packaging process. Common sterilization methods include gamma irradiation or ethylene oxide sterilization.
10. Packaging into Secondary Packaging (if necessary): – Depending on the distribution and storage requirements, blister-packed endodontic NiTi files may be placed into secondary packaging, such as boxes or cartons.
11. Final Inspection: – Perform a final inspection of the packaged endo NiTi files to ensure they meet all quality standards and regulatory requirements.
It’s important to note that specific details of the packaging process may vary based on the design of the blister packaging machine and the requirements of the endodontic NiTi file manufacturer. Following industry standards and regulatory guidelines is essential to ensure the safety, integrity, and quality of the packaged instruments.
Follow us on Facebook: https://www.facebook.com/jidamedtech



